Recent Polarean News and Press Releases

FDA clears XENOVIEW® 3T Chest Coil in GE HealthCare MRI Systems
November 21, 2024
Expands accessibility for institutions utilizing GE HealthCare MRI systems
DURHAM, NC and LONDON, November 21, 2024 (GLOBE NEWSWIRE) – Polarean Imaging plc (AIM: POLX) (“Polarean” or the “Company”), a commercial-stage medical imaging technology leader in advanced Magnetic Resonance Imaging (“MRI”) of the lungs, announces that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the Company’s specialized MRI Chest Coil to now include GE HealthCare 3 Tesla (3T) MRI scanners for the visualization of Xenon-129 nuclei.
Read More
Appointment of Alan Huang, PhD, as Vice President of Sales
September 3, 2024
Experienced business leader with extensive commercial experience in Magnetic Resonance Imaging
DURHAM, NC and LONDON, September 3, 2024 (GLOBE NEWSWIRE) – Polarean Imaging plc (AIM: POLX) (“Polarean” or the “Company”), a commercial-stage medical device leader in advanced Magnetic Resonance Imaging (“MRI”) of the lungs, is pleased to announce the appointment of Alan Huang, PhD as Vice President of Sales. Dr. Huang joins Polarean with nearly 12 years of experience at Philips Healthcare, bringing over 15 years of expertise in the medical device industry and extensive knowledge in MRI and MRI-based technologies.
Read the Full Announcement
Appointment of Chase Hall, M.D., as Chief Medical Advisor
August 28, 2024
Adds significant pulmonary expertise to help drive engagement with the medical community
DURHAM, NC and LONDON, August 28, 2024 (GLOBE NEWSWIRE) – Polarean Imaging plc (AIM: POLX) (“Polarean” or the “Company”), a commercial-stage medical device leader in advanced Magnetic Resonance Imaging (“MRI”) of the lungs, announces that it has appointed Chase Hall, M.D., as Chief Medical Advisor. Dr. Hall is an Associate Professor, Pulmonary, Critical Care and Sleep Medicine, at the University of Kansas Medical Center in the Division of Pulmonary and Critical Care. He also serves as Associate Director at the University of Kansas Interstitial Lung Disease and Rare Lung Disease Clinic. Dr. Hall’s research focus has been on establishing imaging biomarkers in respiratory disease, utilizing Xenon MRI.
Read the Full Announcement
Polarean Raises $12.6 Million in Oversubscribed Financing Round to Accelerate XENOVIEW™ Commercialization and Strategic Growth Initiatives
June 20, 2024
Financing led by existing and new investors to drive commercialization of XENOVIEW
DURHAM, NC and LONDON, June 20, 2024 (GLOBE NEWSWIRE) – Polarean Imaging plc (AIM: POLX) (“Polarean” or the “Company”), a commercial-stage medical device leader in advanced Magnetic Resonance Imaging (MRI) of the lungs, announces the closing of a $12.6 million oversubscribed financing via the Alternative Investment Market (AIM) of the London Stock Exchange. The fundraise was co-led by strategic investors NUKEM Isotopes GmbH and Bracco S.p.A., in addition to support from other existing and new investors.
Read the Full Announcement
Polarean’s Xenon MRI to be Featured at Upcoming ATS 2024 Conference
May 8, 2024
Following the 2024 Respiratory Innovation Summit, the American Thoracic Society 2024 International Conference will witness the number of Xenon MRI displays and the breadth of this research outpace all previous years.
DURHAM, NC and LONDON, May 08, 2024 (GLOBE NEWSWIRE) — Polarean (AIM: POLX), a commercial-stage medical device leader in advanced magnetic resonance imaging (MRI) of the lungs, has been selected as one of the featured companies and a poster presenter at the American Thoracic Society’s (ATS) 2024 Respiratory Innovation Summit (RIS), a testament to the value of our XENOVIEW technology seen across lung clinicians and pulmonary drug developers. The summit is scheduled to take place on May 17th-18th in San Diego, CA, at the Manchester Grand Hyatt San Diego.
Read the Full Announcement
Polarean Partners with VIDA to Streamline Adoption of Advanced MRI of the Lungs
September 8, 2023
Two best-in-class lung imaging companies combine expertise for the deployment of a new xenon 129 MRI platform.
DURHAM, NC and LONDON September 8, 2023 (GLOBE NEWSWIRE) – Polarean (AIM: POLX), a commercial-stage medical device leader in advanced magnetic resonance imaging (MRI) of the lungs, announced today it has partnered with VIDA Diagnostics (VIDA), a clinical imaging intelligence company providing medical imaging software solutions which manage the complexities of digital biomarkers. The companies are partnering to develop solutions that further enable the Polarean xenon 129 MRI platform to accelerate clinical and research use.
Read the Full Announcement
CMS grants reimbursement code for the Polarean XENOVIEW™ MRI Technology
August 29, 2023
Code (C9791) enables healthcare providers a path to bill for “magnetic resonance imaging with inhaled hyperpolarized xenon-129 contrast agent, chest, including preparation and administration of agent”.
DURHAM, NC and LONDON August 29, 2023 (GlobeNewswire) – Polarean (AIM: POLX), a commercial-stage medical device leader in advanced MRI scanning of the lungs, announces that the Centers for Medicare & Medicaid Services (CMS) has established a new reimbursement code for the Polarean XENOVIEW™ (xenon Xe 129, hyperpolarized) technology, effective October 1, 2023.
Read the Full Announcement
Polarean Receives Clearance for New MRI Chest Coil
August 3, 2023
This additional clearance for a new MRI chest coil further supports the recently announced collaboration with Philips.
Polarean (AIM: POLX) (the “Company”), a commercial-stage medical device leader in advanced MRI scanning of the lungs, announces it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the Company’s specialized MRI chest coil to now include Philips 3.0T MRI scanners for the visualization of the Xenon-129 (129Xe) nuclei.
Read the Full Announcement
Appointment of Dr. Christopher von Jako as new CEO
June 21, 2023
Polarean proudly welcomes Christopher von Jako, PhD, as our new Chief Executive Officer and board director
Polarean Imaging plc (AIM: POLX), the medical imaging company, announces that the Company’s Board of Directors has appointed Christopher von Jako, Ph.D. (“Dr. von Jako”) as Chief Executive Officer and director of the Company, effective immediately. Dr. von Jako succeeds Richard Hullihen, who will be retiring and will be stepping down as a Director of the Company, effective immediately. Mr. Hullihen will assist the Company in a transitional position for the next six months.
Read the Full Announcement
Collaboration with Philips to be featured at ISMRM 2023
June 6, 2023
Philips’ MR 7700 MRI scanner to be combined with XENOVIEW™, showcasing the capability to enhance pulmonary imaging by providing regional maps of ventilation in patients’ lungs.
Polarean Imaging plc (AIM:POLX), the medical imaging company, announces that it has entered into a collaboration with Philips, a global leader in health technology, to advance the field of hyperpolarized Xenon MRI. Philips will showcase its 3T MR 7700 system (“MR 7700”), featuring fully integrated multi-nuclei imaging, including Polarean’s XENOVIEW (xenon Xe 129 hyperpolarized) technology at the 2023 International Society for Magnetic Resonance in Medicine Annual Meeting & Exhibition (“ISMRM”), held from 3-8 June, in Toronto, Canada.
Read the Full Announcement
First clinical scan using XENOVIEW™ conducted at Cincinnati Children’s Hospital Medical Center
May 11, 2023
Scan marks a key milestone for imaging of lung ventilation
Polarean Imaging plc’s (AIM:POLX), the medical imaging company, announces that the first clinical scan utilizing its XENOVIEW (xenon Xe 129 hyperpolarized) technology in the United States occurred today at Cincinnati Children’s Hospital Medical Center (“Cincinnati Children’s”). XENOVIEW is the only hyperpolarized contrast agent approved by the U.S. Food and Drug Administration for use with magnetic resonance imaging (MRI) for the evaluation of lung ventilation in adults and pediatric patients aged 12 years and older.
Read the Full Announcement
Polarean to be a featured company at 2023 ATS Respiratory Innovation Summit
May 3, 2023
Polarean also exhibiting at 2023 American Thoracic Society annual meeting, providing a great opportunity to showcase XENOVIEW technology to other pulmonary care innovators and providers
Polarean Imaging plc (AIM: POLX), the medical imaging technology company, has been selected as one of the featured companies as a poster presenter at the American Thoracic Society’s(“ATS”) 2023 Respiratory Innovation Summit (“RIS”), taking place 19-20 May in Washington D.C.
Read the Full Announcement
Company Update
February 16, 2023
Company to focus on commercial sales of XENOVIEWᵀᴹ (xenon Xe 129 hyperpolarized), the first and only FDA approved hyperpolarized MRI contrast agent, and pursue corporate partnering opportunities to drive shareholder value
Polarean Imaging plc (AIM: POLX), the medical imaging technology company, announces that following the approval by the U.S. Food and Drug Administration (“FDA”) for its drug device combination product, XENOVIEW, it intends to pursue a dual strategy of using its current cash resources to maximize commercial sales of XENOVIEW, while also pursuing collaborations with pharmaceutical companies, magnetic resonance imaging (“MRI”) companies, Contract Research Organizations (“CRO”) and other strategic partners to fund the future commercial applications of the Company’s technology.
Read the Full Announcement
FDA Grants New Chemical Entity designation for XENOVIEW™ (xenon Xe 129 hyperpolarized)
February 14, 2023
New Chemical Entity designation from FDA provides a five-year market exclusivity period.
Polarean Inc, (AIM: POLX), the medical imaging technology company, announces that the U.S. Food and Drug Administration (“FDA”) has granted New Chemical Entity (“NCE”) designation for its drug product, XENOVIEW, prepared from the Xenon Xe 129 Gas Blend. XENOVIEW is a hyperpolarized contrast agent indicated for use with magnetic resonance imaging (“MRI”) for evaluation of lung ventilation in adults and pediatric patients aged 12 years and older. XENOVIEW has not been evaluated for use with lung perfusion imaging. It has designated a five-year market exclusivity period.
Read the Full Announcement
FDA Approves Polarean’s XENOVIEW™ (xenon Xe 129 hyperpolarized)
December 28, 2022
XENOVIEW represents the first and only hyperpolarized MRI contrast agent.
FDA approved indication includes both adolescents and adults representing a significant market opportunity.
Polarean Imaging plc (AIM: POLX), the medical imaging technology company, announces that the U.S. Food and Drug Administration (“FDA”) has granted approval for its drug device combination product, XENOVIEW. XENOVIEW, prepared from the Xenon Xe 129 Gas Blend, is a hyperpolarized contrast agent indicated for use with magnetic resonance imaging (“MRI”) for evaluation of lung ventilation in adults and pediatric patients aged 12 years and older. XENOVIEW has not been evaluated for use with lung perfusion imaging.
Read the Full Press Release
Update on New Drug Application
September 22, 2022
U.S. Food and Drug Administration (“FDA”) has requested additional information from Polarean’s xenon-129 gas blend drug manufacturing partner.
Polarean Imaging plc (AIM: POLX), the medical-imaging technology company, with an investigational drug-device combination product using hyperpolarised xenon-129 gas to enhance magnetic resonance imaging (MRI) in pulmonary medicine, announces that the U.S. Food and Drug Administration (“FDA”) has requested additional information from Polarean’s xenon-129 gas blend drug manufacturing partner.
Read the Full Press Release
NDA Resubmission
March 31, 2022
NDA submission for Polarean’s hyperpolarised 129Xenon gas drug-device combination product
Polarean Imaging plc (AIM: POLX), the medical-imaging technology company, with an investigational drug-device combination product using hyperpolarised 129Xenon gas to enhance magnetic resonance imaging (MRI) in pulmonary medicine, announces that the Company has filed the resubmission of its New Drug Application (“NDA”) with the US Food and Drug Administration (“FDA”).
Read the Full Press Release
New System Order
February 22, 2022
Unit order for a Xenon Polariser system from McMaster University in Ontario, Canada.
Polarean Imaging plc (AIM: POLX), the medical-imaging technology company, with an investigational drug-device combination product using hyperpolarised xenon-129 gas to enhance magnetic resonance imaging (MRI) in pulmonary medicine, announces the Company has received an additional research unit order for a Xenon Polariser system from McMaster University in Ontario, Canada. The newest unit (version 9820) will supplement the University’s existing hyperpolarisation 129Xe MRI research programme which is currently using a prior Polarean model (version 9800).
Read the Full Press Release
Complete Response Letter Received from FDA
October 6, 2021
Requirement to address approvability issues identified by FDA ahead of NDA resubmission.
Polarean Imaging plc (AIM: POLX), the medical-imaging technology company, with an investigational drug-device combination product using hyperpolarized xenon-129 gas to enhance magnetic resonance imaging (MRI) in pulmonary medicine, announces that the Company has received a Complete Response Letter (“CRL”) from the U.S. Food and Drug Administration (“FDA”) for the New Drug Application (“NDA”) for their drug-device combination product.
Read the Full Press Release
Polarean Announces NDA Submission of 129Xenon Gas MRI
October 7, 2020
NDA submission for Polarean's hyperpolarized 129Xenon gas drug-device diagnostic for lung imaging.
Polarean Imaging plc (AIM: POLX), the medical‑imaging technology company, with an investigational drug‑device combination product for magnetic resonance imaging (MRI), announces its submission of a New Drug Application (“NDA”) and request for priority review to the US Food and Drug Administration (“FDA”) for hyperpolarized 129Xenon gas used to evaluate pulmonary function and to visualize the lung using MRI.
Read the Full Press Release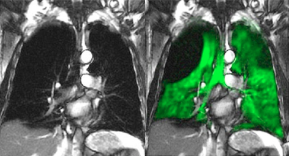
Positive Results Announced from Pivotal Phase III Clinical Trials
January 29, 2020
Both trials met their primary endpoint, showing pre-defined equivalence of hyperpolarized 129Xenon Gas MRI to an approved comparator, 133Xenon Scintigraphy.
Polarean Imaging plc (AIM: POLX), a clinical-stage medical imaging technology company developing a proprietary magnetic resonance imaging (MRI) drug-device combination, today announced positive top-line results from two pivotal Phase III clinical trials of the company’s drug-device combination, which uses hyperpolarized 129Xenon gas MRI to visualize and quantify regional lung function.
The drug, 129Xenon, when polarized in Polarean’s proprietary system, permits functional, regional and quantitative imaging of the lungs using MRI, without the use of ionizing radiation. 129Xenon is administered as an inhaled gas that is given to patients in a 10-second breath-hold procedure. For patients who participated in the clinical trials, the ventilation in zones of interest was quantified and compared to images, similarly quantified, derived from a different imaging modality.
Read the Full Study