Visualization of Lung Function with Xenon MRI
Xenon MRI has the potential to provide a detailed, quantifiable picture of lung imaging, including form and function.
Developing a Tool for the MRI Imaging of Lung Function
The principle behind Xenon MRI technology is simple. A polarization device transforms the inert gas, xenon, into a hyperpolarized state using circularly polarized laser light. This process leaves the gases chemically unchanged, while their nuclei are magnetically aligned. The hyperpolarized gas is inhaled by a patient to fill the space normally occupied by air. The hyperpolarized gas was developed to enhance the MRI signal, making lung function and regional ventilation visible in an MRI scan. Xenon MRI has the potential to enable the study of pulmonary function in ways that are not possible with other lung imaging and diagnostic modalities.
See How Xenon MRI Works
Watch how hyperpolarized xenon-129 gas is inhaled through a single 10-to-15-second breath-hold and distributed to image the smallest airways.
Xenon MRI Development Pipeline
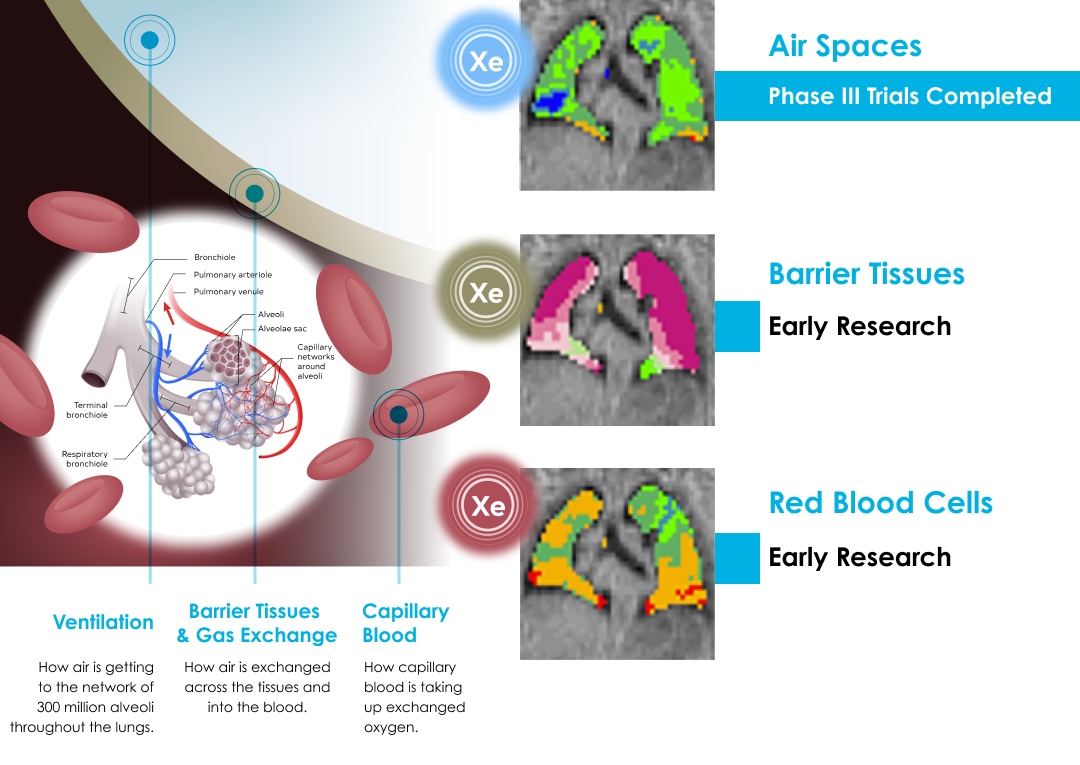
Ongoing Xenon MRI Research
With a visual understanding of lung function, researchers may be able to use Xenon MRI technology to gain new insights into a number of pulmonary diseases and to evaluate new therapeutic agents and procedures. Read about current clinical research into Hyperpolarized Xenon MRI.
